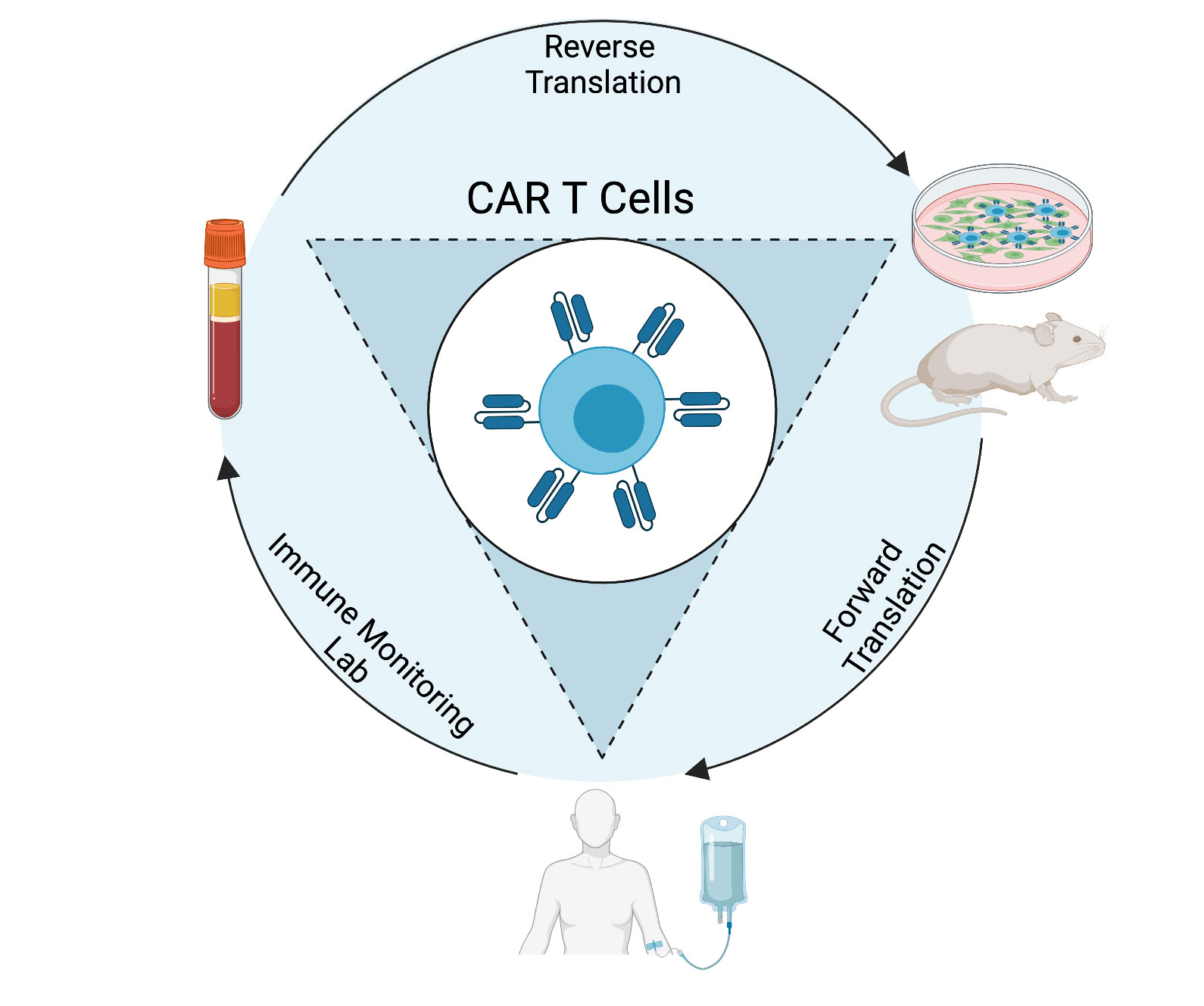
RESEARCH
Overview
Immunotherapy is an effective treatment for patients with certain types of cancer. These innovative treatments use the patient’s immune system to fight off cancer cells. T cells of the immune system are potent pathogen killers and maintain memory to provide long-term protection. We reprogram cancer patients’ T cells to find and kill cancer cells, but not healthy tissue, by expressing a chimeric antigen receptor (CAR) that will bind to tumor-specific antigens on the cancer cell.
The goal of our lab is to improve CAR T cell therapy by making them more effective in killing cancer cells and/or preventing the toxicities associated with treatment.
Our current projects focus on three areas of CAR T cell research.
1. Expanding their use to other tumor types
- Discovering new target antigens, or combinations of antigens, to specifically kill cancer cells but not normal cells.
2. Improving CAR T cell function
- Understanding the basic biology of CAR T cells, and how they interact with cancer cells, to improve their efficacy
3. Developing CAR T cells that are safer for patients
- Manipulating cytokine activity to prevent toxicity
Expanding their use to other tumor types

Created with BioRender.com
The CAR T cells approved by the FDA target CD19 and B cell maturation antigen (BCMA). These CAR T cells work well for treating B cell leukemias and lymphomas and multiple myeloma. However, patients can still relapse with antigen-negative disease, and they do not work for hematologic malignancies that lack CD19 or BCMA expression.
Our lab is working to improve CAR T cells for hematologic malignancies by targeting other or additional antigens beyond CD19 and BCMA that improve tumor cell targeting. We have:
- Developed CAR T cells targeting a novel antigen for B and T cell leukemia and lymphoma, CD37
- Used a natural ligand of BCMA and TACI in its native trimeric form, named TriPRIL, to improve CAR T cell targeting of multiple myeloma
- Combined a novel target antigen, CD79b, with CD19 in a tandem CAR to improve CAR T cell efficacy in B cell leukemia and lymphomas
- Engineered a CD70-targeting CAR that is more stably expressed and, therefore, more effective at targeting acute myeloid leukemia (AML)
- Combined this CD70-CAR with a T cell-engaging molecule (TEAM) targeting CD33 that is secreted from the CAR T cells and activates normal T cells to target AML, named 7033 CAR-TEAM cells
Created with BioRender.com
CAR T cells have been less successful in solid tumors, due to the difficulty in identifying tumor-specific target antigens that will not lead to on-target, off-tumor toxicities. There is also the added difficulty of the tumor microenvironment suppressing CAR T cell function.
To improve CAR T cell targeting of solid tumors, we have:
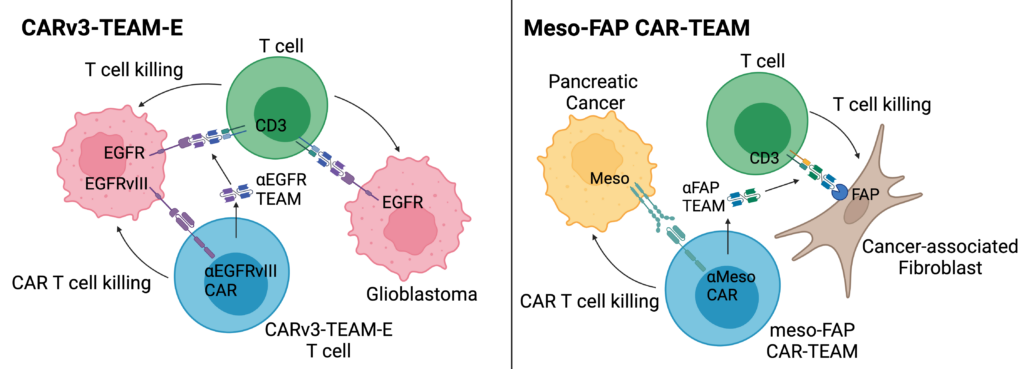
- Engineered CAR T cells to safely target two tumor antigens in glioblastoma with an EGFRvIII-targeting CAR and an EGFR-targeting TEAM that is secreted from the CAR T cell, named CARv3-TEAM-E
- Used a CAR-TEAM system, named meso-FAP CAR-TEAM cells, to better kill pancreatic cancer by targeting a solid tumor antigen (mesothelin – i.e. meso) and an antigen (FAP) expressed by cancer-associated fibroblasts, a cell in the tumor microenvironment that contributes to tumor growth
- Developed CAR T cells targeting a novel solid tumor antigen, MUC17
- Created a tandem CAR against two tumor antigens, Mesothelin and MUC16, to improve targeting of heterogeneous ovarian cancer
- Examined CAR T cells targeting Claudin 18.2 to treat gastric cancers
- Combined solid tumor-targeting CARs with a “bridge” that binds to glycocalyx on the tumor cell surface, increasing the binding affinity between the CAR T cell and tumor cell
Improving CAR T cell function
To make CAR T cells better, we also need to understand exactly how they function. By studying the interactions between CAR T cells and tumor cells and the pathways CAR T cells use to kill tumor cells, we can use this information to optimize their effectiveness.
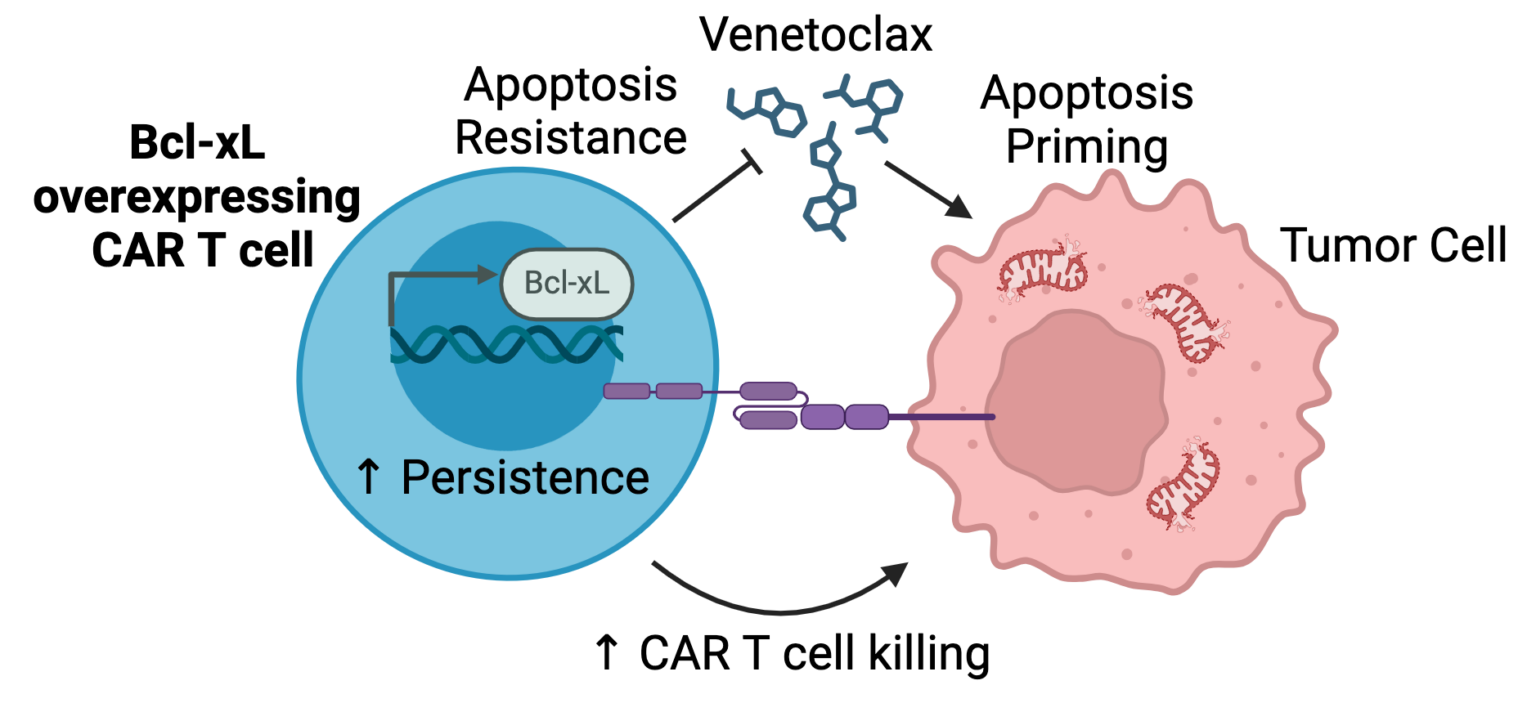
One way we have done this is to understand the cell death pathways that lead to cancer cell killing following interaction with CAR T cells. By understanding these pathways, we can combine CAR T cells with drugs that promote cancer cell death to increase their efficacy. Since the drugs may also kill the CAR T cells in addition to the cancer cells, we can also engineer the CAR T cells to be resistant to the drug and avoid this fate. We recently used this approach to combine venetoclax, a drug that kills cancer cells by inhibiting the anti-cell death protein Bcl-2, with CAR T cells that overexpress another anti-cell death protein, Bcl-xL, to prevent killing the CAR T cells. This combination led to enhanced and sustained killing of tumors in mouse models.
Created with BioRender.com
Developing CAR T cells that are safer for patients
Created with BioRender.com
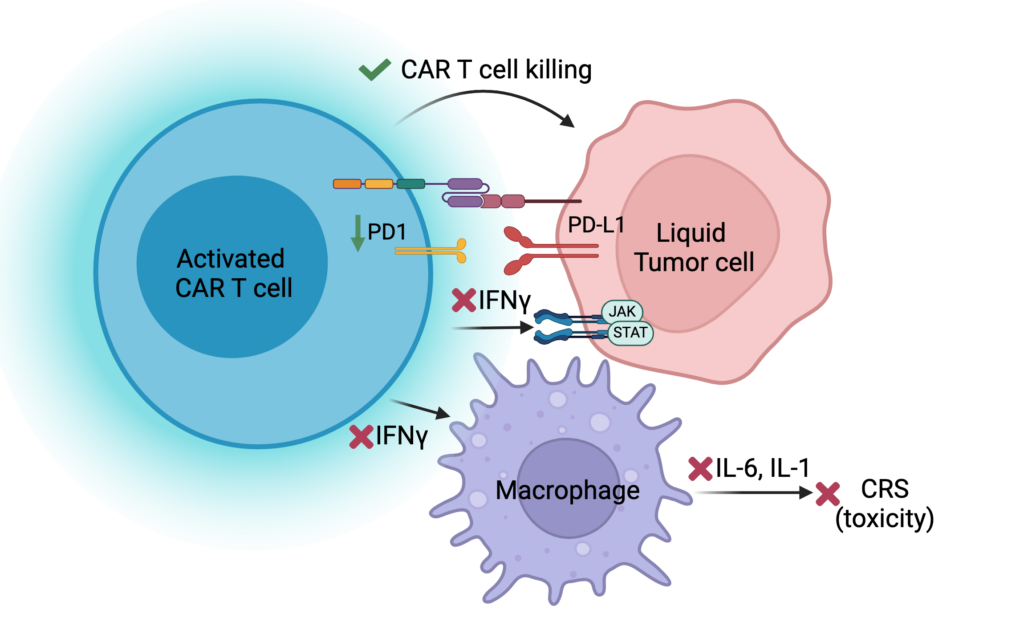
Though CAR T cells work well in some patients, most patients experience toxicity with CAR T cell treatment. These toxicities are caused by the activation of T cells leading to the activation of other immune cells, which release cytokines and cause inflammation. While this process causes toxicity, it was also thought to be essential for CAR T cells to kill tumor cells. We discovered that one cytokine, IFNg, is not necessary for CAR T cells to kill tumor cells but is involved in the first steps of amplifying the cytokines that cause toxicity. Therefore, blocking this cytokine did not impair CAR T cell function but has the potential to prevent toxicity. As a result of this study, we have initiated a clinical trial to treat patients with an anti-IFNg antibody (emapalumab) before they receive their CAR T cells to determine if this will prevent these patients from getting toxicity.
CAR T cells targeting novel antigens
Since the Maus lab was formed in 2015, we have translated several of our novel CAR products to the clinic. Currently, we have three open clinical trials with products we developed and one that is completed. We have also published the initial results from our CARv3-TEAM-E trial for recurrent glioblastoma, where the first three patients had rapid and dramatic regression of their tumors in the days following CAR T cell injection.
Product | Disease | Status | Clinical Trial Registration |
|---|---|---|---|
CAR37 | R/R B & T cell Leukemia and Lymphoma | Completed | NCT04136275 |
TriPRIL | R/R Multiple Myeloma | Recruiting | NCT05020444 |
CARv3-TEAM-E | Glioblastoma | Recruiting | NCT05660369 |
CD79b19 | R/R B cell Leukemia and Lymphoma | Recruiting | NCT06026319 |
R/R, relapsed/refractory
Preventing CAR T cell toxicities
We also have initiated clinical trials to prevent toxicities in patients receiving CD19 CAR T cells. CAR T cell toxicities occur when the T cells become overly activated, leading to the release of inflammatory cytokines. We hypothesize that proactively inhibiting these cytokines could prevent CAR T cell toxicities from occurring. In these trials, we are prophylactically treating patients with anakinra (an IL-1 receptor inhibitor) or emapalumab (an IFNg inhibitor).
Product | Disease | Status | Clinical Trial Registration |
|---|---|---|---|
Axi-cel + anakinra | R/R B cell Leukemia and Lymphoma | Completed | NCT04150913 |
Axi-cel + emapalumab | R/R B cell Lymphoma | Recruiting | NCT06550141 |
Axi-cel, axicabtagene ciloleucel; R/R, relapsed refractory
Our phase I studies will demonstrate the safety of our cellular therapy products in patients, and may provide preliminary efficacy data, but they also provide us with important information on how the CAR T cells behave in patients. Through correlative studies of patient blood and tumor samples throughout the course of their treatment, we can determine how each unique product engrafts, expands, persists, localizes, and functions. This information allows us to understand why a product is (or is not) safe and effective in these patients. Using this knowledge, we can strategically engineer the next generation of CAR T cells that will be safer and more effective in patients. The clinical trial correlative studies are performed by the Immune Monitoring Lab (IML), which is directed by Dr. Kathleen Gallagher.
Created with BioRender.com
Funding
Maus Lab Funding










Trainee Funding